 Nature Communications volume 9, Article number: 3148 (2018)
Nature Communications volume 9, Article number: 3148 (2018)
doi: https://doi.org/10.1038/s41467-018-05593-8
Kamil F. Dziubek, Martin Ende, Demetrio Scelta, Roberto Bini, Mohamed Mezouar, Gaston Garbarino & Ronald Miletich
 The group of ICCOM-CNR and Università degli Studi di Firenze researchers involved in this project. From left to right: Prof. Roberto Bini, Dr. Demetrio Scelta and Dr. Kamil DziubekCarbon dioxide (CO2) is one of the most common components of the Earth’s atmosphere, today mainly known for its major role as a greenhouse gas. Despite the structural simplicity of CO2, its phase diagram currently represents a very controversial issue: a better knowledge of its pressure and temperature behaviour would result in a deeper comprehension of the fundamental properties of the molecule and, from a geophysical and geochemical point of view, of the pivotal role of CO2 in the deep Earth processes.
The group of ICCOM-CNR and Università degli Studi di Firenze researchers involved in this project. From left to right: Prof. Roberto Bini, Dr. Demetrio Scelta and Dr. Kamil DziubekCarbon dioxide (CO2) is one of the most common components of the Earth’s atmosphere, today mainly known for its major role as a greenhouse gas. Despite the structural simplicity of CO2, its phase diagram currently represents a very controversial issue: a better knowledge of its pressure and temperature behaviour would result in a deeper comprehension of the fundamental properties of the molecule and, from a geophysical and geochemical point of view, of the pivotal role of CO2 in the deep Earth processes.
Ranging to extreme pressure (P) and temperature (T) conditions, CO2 shows a very complex structural variability. Below 40 GPa (gigapascal, corresponding to about 0.4 million of atmospheres) several stable and metastable solid molecular phases are reported to exist, all characterized by the presence of linear O=C=O building block mutual interacting through weak van der Waals forces. At higher pressures, instead, CO2 structure undergoes to dramatic changes: as already known for other unsaturated molecules, pressure is in fact an extremely effective tool to induce chemical reactivity and/or polymerization in molecular systems characterized by the presence of multiple bonds. However, bond formation and cleavage present important energetic barriers to be overcome in order to accomplish a chemical transformation: it follows that a CO2 compression at relatively low temperature resulted in the formation of an amorphous material called carbonia, an equivalent of silica glass made up of a network of mixed threefold and fourfold coordinated carbons, representing a kinetically trapped intermediate state. Upon more intense heating, this metastable form converts into an ordered crystalline covalent solid called CO2-V, whose structure resembles that of the β-cristobalite phase of SiO2. The phase V represent the thermodynamically stable phase of CO2 at pressure exceeding 50 GPa (or, in other words, over 0.5 million of atmospheres).
 The Raman setup at LENS, used for the spectroscopic characterization of the CO2 sample at megabar pressures. Photo taken by Andrea Taschin.We report the first experimental study of the CO2 behaviour at P,T conditions similar to those of the core-mantle boundary (CMB), where only computational studies were previously performed. In fact, older experimental studies attained a range of pressure up to a maximum of 70 GPa, reporting on the CO2 dissociation in carbon (diamond) and red oxygen (the ε phase of molecular solid oxygen, consisting of O8 units). These observations would have profound implications related to oxygen fugacity in the deep mantle, to the oxidation of the surrounding minerals and to the formation of superdeep diamonds. On the contrary, other experiments showed the formation of novel, exotic ionic phases of CO2. All these observations were in stark contrast to the most recent theoretical computations, that attested for a substantial stability of CO2 against dissociation up to 200 GPa and 10000 K.
The Raman setup at LENS, used for the spectroscopic characterization of the CO2 sample at megabar pressures. Photo taken by Andrea Taschin.We report the first experimental study of the CO2 behaviour at P,T conditions similar to those of the core-mantle boundary (CMB), where only computational studies were previously performed. In fact, older experimental studies attained a range of pressure up to a maximum of 70 GPa, reporting on the CO2 dissociation in carbon (diamond) and red oxygen (the ε phase of molecular solid oxygen, consisting of O8 units). These observations would have profound implications related to oxygen fugacity in the deep mantle, to the oxidation of the surrounding minerals and to the formation of superdeep diamonds. On the contrary, other experiments showed the formation of novel, exotic ionic phases of CO2. All these observations were in stark contrast to the most recent theoretical computations, that attested for a substantial stability of CO2 against dissociation up to 200 GPa and 10000 K.
We performed our experiment at pressure ranges from 85 to 120 GPa (so up to a maximum of 1.2 million of atmospheres) and at temperature of about 2700 K. A small sample of CO2 (40 microns in diameter, about a twentieth of a millimetre) was studied in a diamond anvil cell (DAC), a device that allows to generate continuous and static pressure on small volume samples. The sample was compressed at room temperature up to 85 GPa, well beyond the pressure threshold where the amorphous carbonia is formed, and then it was heated up to 2700 K using the laser heating technique. Laser heating allows to perform intense heating of small samples through the absorption of the radiation produced by a high power laser (i.e. a CO2 laser). The heating immediately transformed the amorphous phase into the crystalline phase CO2-V, without any evidence of CO2 dissociation into its elemental constituents or ionic species formation. Further heating at higher pressure had the effect of release the residual strain in the crystal, without significantly altering its structure. The sample was characterized by means of X-ray diffraction techniques at the ESRF in Grenoble, computing its experimental equation of state, and then by means of Raman spectroscopy at LENS.
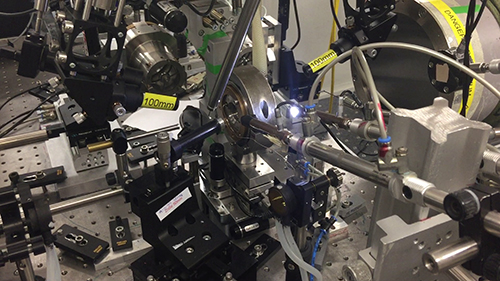 The diamond anvil cell (DAC) used to compress the sample up to 120 GPa (about 1.2 million atmosphere) and the laser heating setup to heat it to 2700 K (about 2400 Celsius degrees). This experimental study allows to extend the stability range of CO2-V up to P,T conditions similar to those of the core-mantle boundary, thus updating the whole phase diagram of this molecule in the region of existence of its covalent extended solid phases and confirming how the phase V is in fact the only thermodynamically stable phase of CO2 in conditions relevant to the CMB and, by extension, to the deep Earth interiors. This finding is extremely important to clarify the pivotal role of CO2 in the framework of deep carbon cycle. A great amount of CO2 reaches the Earth interiors through subductive slabs movements, but just a relatively small part of it comes out to the atmosphere through the volcanic emissions. Taking into account our experimental results, CO2 would not decompose inside the mantle (as it was believed to) but instead it could remain stable adopting the CO2-V structure. This obviously represents a key aspect for the possible chemical reactivity of CO2 towards the surrounding minerals that complicate the speciation and the distribution of mantle carbon materials.
The diamond anvil cell (DAC) used to compress the sample up to 120 GPa (about 1.2 million atmosphere) and the laser heating setup to heat it to 2700 K (about 2400 Celsius degrees). This experimental study allows to extend the stability range of CO2-V up to P,T conditions similar to those of the core-mantle boundary, thus updating the whole phase diagram of this molecule in the region of existence of its covalent extended solid phases and confirming how the phase V is in fact the only thermodynamically stable phase of CO2 in conditions relevant to the CMB and, by extension, to the deep Earth interiors. This finding is extremely important to clarify the pivotal role of CO2 in the framework of deep carbon cycle. A great amount of CO2 reaches the Earth interiors through subductive slabs movements, but just a relatively small part of it comes out to the atmosphere through the volcanic emissions. Taking into account our experimental results, CO2 would not decompose inside the mantle (as it was believed to) but instead it could remain stable adopting the CO2-V structure. This obviously represents a key aspect for the possible chemical reactivity of CO2 towards the surrounding minerals that complicate the speciation and the distribution of mantle carbon materials.
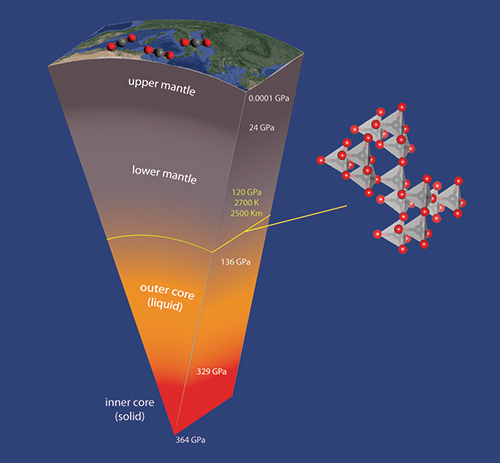 Temperature and pressure variation with depth in the Earth’s interiors. Experimental conditions for our study are highlighted by the yellow line (with indication of pressure, temperature and depth). On the right, the structure of CO2-V, found to be stable at this P,T conditions.This study involved three researchers from Firenze, Demetrio Scelta (post-doc researcher at ICCOM-CNR), Kamil Dziubek (post-doc researcher at ICCOM-CNR) and Prof. Roberto Bini (Università degli Studi di Firenze, ICCOM-CNR associate). The research has been performed thanks to the collaboration of the European Laboratory for Non-linear Spectroscopy (LENS, Firenze), University of Vienna and the high-pressure dedicated beamline ID27 at the ESRF synchrotron in Grenoble, France. The success of this scientific project attests again the importance of the collaboration and synergy that involves, since many years, the structures and researcher from ICCOM-CNR, DSCTM-CNR and LENS.
Temperature and pressure variation with depth in the Earth’s interiors. Experimental conditions for our study are highlighted by the yellow line (with indication of pressure, temperature and depth). On the right, the structure of CO2-V, found to be stable at this P,T conditions.This study involved three researchers from Firenze, Demetrio Scelta (post-doc researcher at ICCOM-CNR), Kamil Dziubek (post-doc researcher at ICCOM-CNR) and Prof. Roberto Bini (Università degli Studi di Firenze, ICCOM-CNR associate). The research has been performed thanks to the collaboration of the European Laboratory for Non-linear Spectroscopy (LENS, Firenze), University of Vienna and the high-pressure dedicated beamline ID27 at the ESRF synchrotron in Grenoble, France. The success of this scientific project attests again the importance of the collaboration and synergy that involves, since many years, the structures and researcher from ICCOM-CNR, DSCTM-CNR and LENS.


